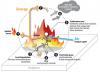
Flame retardation in general
Flame retardants decrease the ignitability of materials and inhibit the combustion process, limiting the amount of heat released. The simplest way, in theory, of preventing polymer combustion is to design the polymer so that it is thermally stable. Thermally stable polymers are less likely to decompose into combustible gases under heat stress, which prevents combustion from initiating. Because thermally stable polymers are often difficult and expensive to process and may have performance limitations, manufacturers use other means, such as flame-retardant chemicals, to impart flame-retardant properties to polymers.
Flame retardants decrease the likelihood of a fire occurring and/or decrease a range of undesirable consequences of a fire (Lyons 1970; Cullis and Hirschler 1981). However, in other instances the incomplete combustion resulting from the use of flame retardants, where oxidation and/or thermal transfer are inhibited, can produce negative by-products. Carbon monoxide (CO), a by-product of incomplete combustion, acts as an asphyxiant in poorly-ventilated fire scenarios and can lead to CO poisoning and death (Nelson 1998; Peck 2011). These by-products are in addition to the production of other toxic chemicals (e.g., halogenated dioxins and furans) generated during combustion of materials containing flame retardants.
Fire occurs in three stages: (1) thermal decomposition, where the solid, or condensed phase, breaks down into gaseous decomposition products as a result of heat; (2) combustion chain reactions in the gas phase, where thermal decomposition products react with an oxidant (usually air) and generate more combustion products, which can then propagate the fire and release heat; and (3) transfer of the heat generated from the combustion process back to the condensed phase to continue the thermal decomposition process (Hirschler 1992; Beyler and Hirschler 2002).
The basic mechanisms of flame retardancy will vary depending on the flame retardant and polymer system. Flame retardants can be classified based on the phase (solid or gas) in which they act to reduce or prevent propagation of flame. Other flame retardants may form protective barriers over a polymer which may insulate the flammable polymer from heat or reduce the amount of polymer that is available to burn as fuel. In either state, gaseous or condensed, flame retardants will act to decrease the release rate of heat (Hirschler 1994), thus reducing the burning rate, flame spread, and/or smoke generation (Morose 2006).
Typically, flame retardants contain one or more of the following elements: chlorine, bromine, aluminum, boron, nitrogen, phosphorus, or silicon (Lyons 1970; Cullis and Hirschler 1981). There are a number of alternatives and synergists that are also effective. Some elements, such as zinc (often used as zinc borate or zinc stannate) and molybdenum (often used as ammonium molybdates), are effective primarily as smoke suppressants in mixtures of flame retardants. Antimony trioxide can serve as an effective synergist in combination with halogenated flame retardants.
The amount of flame retardant needed to pass a given flammability standard varies due to a number of factors. In general, the lowest levels of flame retardants are required with bromine-based chemistries, and higher levels are required when using mineral-type compounds. Ranges of typical “loading levels” (how much of a flame retardant is added to a material) for common flame retardants are shown in Table 3-1. Loading levels also depend on the polymers in which the flame retardant is used. For example, bromine-based flame retardants are used in a wide variety of products (e.g., polyolefins, styrene, polyamides (PAs), polyesters, polycarbonates (PCs) and textiles) and thus have a wide range of loading levels9. This is demonstrated by the fact that when used in polyesters, bromine-based flame retardants have a loading level of about 8 percent, whereas when bromine-based flame retardants are used in textiles, they are usually at about a 17 percent loading. On the other hand, the flame retardants that are not used in such a wide variety of products have much smaller loading ranges. For example, chlorophosphates have a 9 percent loading in epoxy resins and a 10 percent loading in polyurethane and are not reportedly used with other polymers (Weil and Levchik 2009).
Typical Loading Levels of Common Flame Retardants
Type of Flame Retardant | Loading (wt %) |
Bromine-based | 2 to 25%1 |
Aluminum Hydroxide | 13 to 60% |
Magnesium Hydroxide | 53 to 60% |
Chlorophosphates | 9 to 10% |
Organophosphorus | 5 to 30% |
1Polyethylene (PE) can require up to 31% of a bromine based flame retardant and 7‒8 % antimony trioxide. However, this is rarely practiced in the market thus the upper limit displayed above is 25%. Source: Weil and Levchik 2009 | |
Flame-Retardant Classification
Flame retardants can be classified into four main categories according to chemical composition:
Inorganic: This category includes flame retardants and synergists such as silicon dioxide, metal hydroxides (e.g., aluminum hydroxide and magnesium hydroxide), antimony compounds (e.g., antimony trioxide), boron compounds (e.g., zinc borate – which is often used as a synergist for both halogenated and non-halogenated flame retardants), and other metal compounds (molybdenum trioxide). As a group, these flame retardants represent the largest fraction of total flame retardants in use (Norwegian Pollution Control Agency 2009).
Halogenated: These flame retardants are primarily based on bromine and chlorine. Typical halogenated flame retardants are halogenated paraffins, halogenated aliphatic and aromatic compounds, and halogenated polymeric materials. Some halogenated flame retardants also contain other elements, such as phosphorus or nitrogen. The effectiveness of halogenated additives, as discussed below in Section 3.4, is due to their interference with volatile substances which are created in the combustion process, decreasing their combustibility. Brominated compounds represent approximately 18 to 21 percent (by volume) of the global flame-retardant production (Hirschler 1998).
Phosphorus-based: This category represents about 20 percent (by volume) of the global production of flame retardants and includes organic and inorganic phosphates, phosphonates, and phosphinates as well as red phosphorus, covering a wide range of phosphorus compounds with different oxidation states. There are also halogenated phosphate esters, often used as flame retardants for polyurethane foams or as flame-retardant plasticizers, but not commonly used in electronics applications (Hirschler 1998; Green 2000; Weil and Levchik 2004).
Nitrogen-based: These flame retardants include melamine and melamine derivatives (e.g., melamine cyanurate, melamine polyphosphate). Nitrogen-containing flame retardants are often used in combination with phosphorus-based flame retardants, with both elements in the same molecule (Morose 2006).
Halogenated flame retardants are commonly blended with a synergist, such as antimony trioxide. A synergist multiplicatively enhances the flame retardant effect. Many flame-retardant synergists do not have significant flame-retardant properties by themselves; their addition increases the overall effectiveness of the flame-retardant system. It should also be noted that the synergists may be very system specific; they are not universal. For example, antimony trioxide only shows flame retardant synergism with halogenated flame retardants and has no effect when combined with inorganic, phosphorus, or nitrogen-based flame retardants.
Flame retardants also can be classified by how they are incorporated into a polymer – additively or reactively. No reactive-type flame retardants were identified as alternatives to decabromodiphenyl ether (decaBDE) in this assessment.
Additive: Additive flame retardants are incorporated into polymers via physical mixing, and are not chemically bound to the polymer. Flame-retardant compounds are mixed with existing polymers without undergoing any chemical reactions. As a result, the polymer/additive mixture is less susceptible to combustion than the polymer alone. Since additive flame retardants can be incorporated into the product up until the final stages of manufacturing, it is usually easier for manufacturers to use additive flame retardants than reactive flame retardants.
Reactive: Reactive flame retardants are incorporated into polymers via chemical reactions and must be incorporated at an early stage of manufacturing. Once introduced, they become a permanent part of the polymer structure – i.e., the chemically-bound reactive flame-retardant chemicals cease to exist as separate chemical entities. As a result, reactive flame retardants have a greater effect on the chemical and physical properties of the polymer into which they are incorporated than do additive flame retardants. For examples of reactive flame retardants, refer to the Flame Retardants in Printed Circuit Boards Draft Report (U.S. EPA 2008).
Flame retardants can also be coated on the external surface of the polymer to form a protective barrier or to improve their compatibility with the polymeric matrix.
Both reactive and additive flame retardants can significantly change the properties of the polymers into which they are incorporated. Each flame retardant polymer combination is unique. For example, they may change the viscosity, flexibility, density, electrical properties, tensile strength, and flexural strength; and may also increase the susceptibility of the polymers to photochemical and thermal degradation.
Alternatives and substituents for decaDBE evaluated in the US EPA study (see source)
Aluminum Diethylphosphinate
Aluminum Hydroxide
Ammonium Polyphosphate
Antimony Trioxide
Bis(hexachlorocyclopentadieno) Cyclooctane
Bisphenol A Bis-(diphenyl phosphate), BAPP
Brominated Epoxy Polymers
Brominated Epoxy Polymer(s)
Mixture of Brominated Epoxy Polymer(s) and Bromobenzyl Acrylate
Brominated Epoxy Resin End-Capped with Tribromophenol
Brominated Polyacrylate
Brominated Poly(phenylether)
Brominated Polystyrene
Decabromodiphenyl Ethane
Decabromodiphenyl Ether
Ethylene Bis-Tetrabromophthalimide (EBTBP)
Magnesium Hydroxide
Melamine Cyanurate
Melamine Polyphosphate
N-alkoxy Hindered Amine Reaction Products
Phosphonate Oligomer
Phosphoric acid, mixed esters with [1,1'-bisphenyl-4,4'-diol] and phenol; BPBP
Polyphosphonate
Poly[phosphonate-co-carbonate]
Red Phosphorus
Resorcinol Bis-Diphenylphosphate; RDP
Substituted Amine Phosphate Mixture
Tetrabromobisphenol A Bis (2,3-dibromopropyl) Ether
Triphenyl Phosphate
Tris(tribromoneopentyl) Phosphate
Tris(tribromophenoxy) Triazine
Zinc Borate
The dateiled assessment of these possible flame retardants can be found in the US EPA szudy given as the source of this short overview.
Source: US EPA (2014) An alternatives assessment for the flame retardant decabromodiphenyl ether (decaBDE) Online available from: http://www.epa.gov/dfe/pubs/projects/decaBDE/deca-report-complete.pdf
US EPA (2014) An alternatives assessment for the flame retardant decabromodiphenyl ether (decaBDE) Online available from: http://www.epa.gov/dfe/pubs/projects/decaBDE/deca-report-complete.pdf