CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
Chemical name |
Ethanol [1] |
|
Synonyms |
ethyl alcohol [1] |
|
IUPAC name |
ethanol [1] |
|
CAS No |
64-17-5 |
|
REACH registration number |
|
|
EC No |
200-578-6 |
|
Molecular formula |
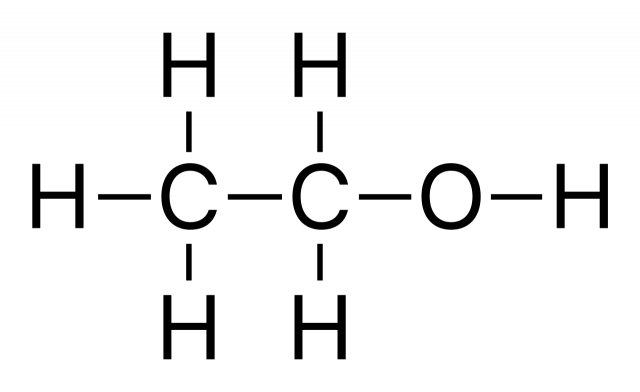
C2H6O [1] |
|
Substance group/chemical family |
Mono constituent substance/Organic/Alcohol [1] |
|
Appearance Physical state Odour Form Colour |
Liquid (100%) [1]
Other (75%), Pungent (25%) [1]
colourless |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
This substance is used in the following products: coating products, fuels, washing & cleaning products, inks and toners, anti-freeze products, adhesives and sealants, non-metal-surface treatment products, heat transfer fluids, polishes and waxes, extraction agents, textile treatment products and dyes, fillers, putties, plasters, modelling clay, metal surface treatment products, leather treatment products and photo-chemicals. This substance has an industrial use resulting in manufacture of another substance (use of intermediates).This substance is used for the manufacture of: chemicals, machinery and vehicles, textile, leather or fur, furniture, electrical, electronic and optical equipment and fabricated metal products.[1] It is used in alcoholic beverages in suitable dilutions, and as a reagent in synthetic organic chemistry and chromatography, as well as industrial and laboratory organic solvent. Other uses are in manufacture of denatured alcohol, pharmaceuticals (rubbing compounds, lotions, tonics, colognes), in perfumery. Octane booster in gasoline. Pharmaceutic aid (solvent). [3] |
|
Handling considerations |
Prevention statementsWhen handling this substance: do not handle until all safety precautions have been read and understood; ground and bond container and receiving equipment; take actions to prevent static discharges; do not breathe the dust, fume, gas, mist, vapours or spray; keep away from heat, sparks, open flames and/or hot surfaces – No smoking; obtain special instructions before use; keep container tightly closed; do not eat, drink or smoke when using this product; use explosion-proof equipment (electrical/ventilating/lighting/etc.); use non-sparking tools; wash parts of the body (as specified by manufacturer/supplier)in contact with substance thoroughly after handling; wear protective gloves and/or clothing, and eye and/or face protection as specified by manufacturer/supplier. Response statementsIn case of incident: If exposed or concerned: get medical advice/attention. If on skin (or hair): take off immediately all contaminated clothing. Rinse skin with water or shower. In case of fire: Use (measures specified by manufacturer/supplier) for extinction. If in eyes: rinse cautiously with water for several minutes. Remove contact lenses if present and easy to do – continue rinsing. Immediately call a poison center or doctor/physician. If eye irritation persists get medical advice/attention. Rinse the mouth. If swallowed: call a poison center or doctor/physician if you feel unwell. If on skin: wash with soap and water. Storage statementsStore this substance in a well-ventilated place and Keeping it cool; locked up; in a well-ventilated place and keeping container tightly closed. Disposal statementsThe substance must be disposed in accordance with local/regional/national/international regulation [1] |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
46.07 g/mol [2] |
|
Bulk density/Specific gravity |
0.785 - 0.789 g/cm³ @ 20 - 25 °C [1] |
|
pH |
|
|
Particle size |
|
|
EC |
1.35X10-9/ohm cm at 25 °C [3] |
|
Melting/Freezing point |
-114.49 - -97.8 °C @ 101.325 kPa [1] |
|
Boiling point |
78.29 - 78.293 °C [1] |
|
Flash point |
12.85 - 13 °C at 101 325 Pa [1] |
|
Flammability |
Highly flammable (100%) [1] |
|
Vapour density |
1.59 (Air = 1) [3] |
|
Vapour pressure |
57.26 hPa @ 19.6 - 19.65 °C [1] |
|
Solubility in water |
789 g/L @ 20 °C [1] Soluble in water in all proportions. [3] |
|
Solubility in organic solvents |
Miscible with ethyl ether, acetone, chloroform; soluble in benzene [3] |
|
Solubility in inorganic solvents |
|
|
Hydrolysis |
Hydrolysis is not expected to be an important environmental fate process since this compound lacks functional groups that hydrolyze under environmental conditions (pH 5 to 9). [3] |
|
Ionicity in water |
|
|
Surface tension |
21.97 mN/m at 25 °C [3] |
|
Dispersion properties |
|
|
Explosiveness |
Non-explosive (100%) [1] |
|
Other properties |
Autoflammability / self-ignition at 101 325 Pa: 362.85 - 368.8 °C [1] Non-oxidising (100%) [1] Dynamic viscosity at 20 °C: 1.17 - 1.2 mPa.s [1] |
|
Stability and reactivity |
|
|
Chemical stability |
Stable under recommended storage conditions. [3] |
|
Reactivity hazards |
|
|
Corrosivity |
|
|
Polimerization |
|
|
Incompatibility with various substances |
Alkali metals, oxidizing agents, peroxides. [3] |
|
Special remarks on reactivity |
Reactive groups: Alcohols and Polyols Esters, Sulfate Esters, Phosphate Esters, Thiophosphate Esters, and Borate Esters [3] |
|
Physical, chemical and biological coefficient |
|
|
Koc |
log Koc: 0.20 [3] |
|
Kow |
Log Kow (Log Pow): -0.35 @ 20 - 24 °C [1] |
| logKoa | 3.25 (Octanol-Air partition coefficient) [3] |
|
pKa |
15.8 at 20 °C [1] |
|
log Kp |
|
|
Henry-constant |
5X10-6 atm-cu m/mol at 25 °C[3] |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
|
|
Artificial pollution sources |
Ethanol's production and use as a biofuel or biofuel additive, in alcoholic beverages, as a solvent, in pharmaceuticals, perfumery and organic synthesis may result in its release to the environment through various waste streams. It's use as a pesticide (disinfectant, sanitizer, microbiocide, fungicide, plant regulator) will result in its direct release to the environment. Ethanol has been detected in emissions from animal wastes, plants, insects, forest fires, microbes, and volcanoes as well as from the natural fermentation of starch, sugar and other carbohydrates. Ethanol is a volatile component of many plants. [3] |
|
General terrestrial fate |
Ethanol is expected to have very high mobility in soil. Volatilization of ethanol from moist soil surfaces is expected given a Henry's Law constant of 5.0X10-6 atm-cu m/mole. Ethanol is expected to volatilize from dry soil surfaces based upon a vapor pressure of 59.3 mm Hg at 25 °C. Ethanol, present at 100 mg/L, reached 89% of its Theoretical BOD in 2 weeks using an activated sludge inoculum at 30 mg/L in the Japanese MITI test. Ethanol, present at 100 mg/L, was completely degraded in 5-8 days in an aerobic sandy soil/groundwater microcosm [3]. |
|
General aquatic fate |
Ethanol is not expected to adsorb to suspended solids and sediment. Volatilization from water surfaces is expected based upon a Henry's Law constant of 5.0X10-6 atm-cu m/mole. Using this Henry's Law constant and an estimation method, volatilization half-lives for a model river and model lake are 5 and 39 days, respectively. Ethanol is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions. According to a classification scheme, an estimated BCF of 3, from its log Kow of -0.31 and a regression-derived equation, suggests the potential for bioconcentration in aquatic organisms is low. Biodegradation of ethanol in water is expected based on degradation half-lives on the order of a few days in aquatic studies conducted using microcosms constructed with a low organic sandy soil and groundwater [3]. |
|
General atmospheric fate |
According to a model of gas/particle partitioning of semivolatile organic compounds in the atmosphere, ethanol, which has a vapor pressure of 59.3 mm Hg at 25 °C, is expected to exist solely as a vapor in the ambient atmosphere. Vapor-phase ethanol is degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 5 days, calculated from its rate constant of 3.27X10-12 cu cm/molecule-sec at 25 °C. Ethanol does not contain chromophores that absorb at wavelengths >290 nm and, therefore, is not expected to be susceptible to direct photolysis by sunlight [3]. |
|
General persistence and degradability |
|
|
Abiotic degradation and metabolites |
The rate constant for the vapor-phase reaction of ethanol with photochemically-produced hydroxyl radicals is 3.27X10-12 cu cm/molecule-sec at 25 °C. This corresponds to an atmospheric half-life of about 5 days at an atmospheric concentration of 5X10+5 hydroxyl radicals per cu cm. Ethanol is not expected to undergo hydrolysis in the environment due to the lack of functional groups that hydrolyze under environmental conditions. Ethanol does not contain chromophores that absorb at wavelengths >290 nm and, therefore, is not expected to be susceptible to direct photolysis by sunlight. A smog chamber test with 2 ppm ethanol and 1 ppm nitrogen resulted in 20% degradation in 5 hr. Ethanol is considered to have low reactivity in photochemical smog. Reaction with hydroxyl radicals in aquatic media will not likely be an important environmental process. [3] |
|
Biodegradation and metabolites |
Readily biodegradable in soil and water under aerobic and aanerobic conditins (100%) [1,3] Ethanol was shown to biodegrade under aerobic conditions in various screening tests using different types of inocula and incubation periods(1-8). [3] Ethanol rapidly degraded in soil forming formaldehyde and acetic acid along with carbon dioxide and water. [3] At a starting concentration of 100 mg/L, ethanol was rapidly degraded in anaerobic microcosms prepared from low organic (0.2% organic carbon) sandy aquifer material and ground water at pH 5.2 with a half-life of approximately 1.5 days under denitrifying conditions and about 5 days under iron-reducing conditions. [3] |
|
Bioconcentration |
An estimated BCF of 3 was calculated in fish for ethanol, using a log Kow of -0.31 and a regression-derived equation. According to a classification scheme, this BCF suggests the potential for bioconcentration in aquatic organisms is low. [3] |
|
Volatilization |
Henry's Law constant indicates that ethanol is expected to volatilize from water surfaces. Based on this Henry's Law constant, the volatilization half-life from a model river (1 m deep, flowing 1 m/sec, wind velocity of 3 m/sec) is estimated as 5 days. The volatilization half-life from a model lake (1 m deep, flowing 0.05 m/sec, wind velocity of 0.5 m/sec) is estimated as 39 days. Ethanol's Henry's Law constant indicates that volatilization from moist soil surfaces may occur. Ethanol is expected to volatilize from dry soil surfaces based upon an extrapolated vapor pressure of 59.3 mm Hg. [3] |
|
Photolysis |
Phototransformation in air: Half life in air: 38 h [1] Ethanol does not contain chromophores that absorb at wavelengths >290 nm and, therefore, is not expected to be susceptible to direct photolysis by sunlight. [3] |
|
Hydrolysis |
Hydrolysis is not expected to be an important environmental fate process since this compound lacks functional groups that hydrolyze under environmental conditions (pH 5 to 9). [3] |
|
Soil adsorption and mobility |
Soil Adsorption Coefficient: 1.58 L/kg [3] A log Koc of 0.20 has been reported for ethanol. According to a classification scheme, this Koc value suggests that ethanol is expected to have very high mobility in soil. Transport was not retarded in an ethanol leaching test using a shallow sand and gravel test aquifer in Merrick Co, central Platte Valley, Nebraska. A sorption coefficient on a snow surface was reported as log K = -3.04 (cu m snow surface/sq m air) at -6.8 °C. [3] |
|
ENVIRONMENTAL CONCENTRATIONS |
|
|
Measured data |
% Distribution in Media: Air: 12.5 - 73.3 % Water: 15.6 - 87.5 % Soil: 0 - 11.1 % Sediment: 0 - 0.02 % Suspended sediment: 0 % Biota: 0% Aerosol: 0% [1] |
|
ECOTOXICOLOGICAL INFORMATION |
|
|
General adverse effects on ecosystem |
|
|
Acute toxicity (LC50, EC50) |
|
|
Aquatic systems |
LC50: 11200 - 14200 mg/L (freshwater fish, 4 days) [1] EC50 / LC50: 5012 mg/L (freshwater invertebrates) [1] EC50 / LC50: 857 mg/L (marine water invertebrates) [1] EC50: 275 mg/L (freshwater algae) [1] EC50: 1900 - 1970 mg/L (marine water algae) [1] EC50: 4432 mg/L (freshwater plants) [1] EC50: 5800 mg/L (microorganisms) [1] |
|
Terrestrial systems |
EC50 / LC50: 633 mg/kg soil dw (terrestrial plants) [1] |
|
Chronic toxicity (NOEC, LOEC) |
|
|
Aquatic systems |
EC10 / LC10 or NOEC: 250 mg/L (freshwater fish) [1] EC10 / LC10 or NOEC: 9.6 mg/L (freshwater invertebrates) [1] EC10 / LC10 or NOEC: 79 mg/L (marine water invertebrates) [1] EC10 or NOEC: 11.5 mg/L (freshwater algae) [1] EC10 or NOEC: 1580 mg/L (marine water algae) [1] EC10 or NOEC: 280 mg/L (freshwater plants) [1] |
|
Terrestrial systems |
|
|
HUMAN HEALTH EFFECTS and PROTECTION |
|
|
Routes of human exposures |
Occupational exposure to ethanol may occur through inhalation and dermal contact with this compound at workplaces where ethanol is produced or used. The general population is exposed to ethanol primarily through the consumption of alcoholic beverages containing this product. Monitoring data indicate that the general population may also be exposed to ethanol via inhalation of ambient air or dermal contact with products containing ethanol. [3] |
|
General effects |
irritation eyes, skin, nose; headache, drowsiness, lassitude (weakness, exhaustion), narcosis; cough; liver damage; anemia. [3] |
|
Endocrine disruption |
Immunotoxicity: No adverse effect observed (subchronic, rat, inhalation route) [1] No evidence to suggest adverse effects occur at a level that would trigger classification. [2] |
|
Mutagenicity |
There is no significant evidence that ethanol is a genotoxic hazard according to the criteria applied normally for the purposes of classification and labelling, when data that is only applicable to heavy consumption of alcoholic beverages is excluded, the limit doses normally applied in guideline studies are taken into consideration and the fact that confounding due to other toxic effects associated with very high doses are accepted. [2] |
|
Carcinogenicity |
There is no significant evidence to warrant a classification of ethanol for cancer in the context of the relevant classification and labelling regulations for chemical substances. [2] The International Life Sciences Institute published an extensive review of the health issues relating to alcohol consumption (“Health issues relating to alcohol consumption”, ILSI, 1999) and concluded that ethanol is not a carcinogen by standard laboratory tests using animals. In humans, the only epidemiology data that could be relevant to the hazard presented through the use of ethanol as a chemical substance at the workplace and in consumer products is that relating to breast cancer. However, the available data shows that, at the doses feasible from such uses, even over a lifetime, there is no sound evidence of any breast cancer hazard. Even the studies that do conclude a risk suggest that the increased rate is small and that has to be put into context of the relatively high rate of breast cancer incidence in the female population and the likelihood of other factors possibly confounding the findings. [2] According to PubChem [3] ethanol is a Confirmed animal carcinogen with unknown relevance to humans, based on [4]. |
|
Reprotoxicity |
No adverse effect observed (subchronic, mouse, oral route) effect on fertility [1] No adverse effect observed subchronic, rat, inhalation route) developmental toxicity [1] Overall, it can be concluded that adverse effects from the effects of ethanol treatment are only seen at very high doses only relevant to deliberate and repeated oral consumption of ethanol. The most important studies are the 2-generation study which shows a NOAEL of 13.8g/kg and the inhalation studies that show a NOAEC of 16000ppm (the maximum tested exposure, which is close to or exceeding 50% of the lower explosive limit.) On this basis, it can be concluded that it is impossible to reach the doses of ethanol required to produce any sort of adverse reproductive response other than by repeated oral consumption of large amounts of ethanol, doses normally only associated with problem drinking, and therefore classification for reproductive or developmental toxicity in the context of a chemical substance is not appropriate or warranted. [2] |
|
Teratogenicity |
Prenatal ethanol exposure affected fetal skeletal ossification at exposure levels lower than those required to affect fetal body weight and length, although the significance of these changes for long-term bone health is unknown. No statistically significant effects on fetal growth or ossification were seen in the fetuses of dams ingesting about 5.2 g ethanol/kg bw/day from 3 weeks prior to mating to GD 21. [2] The data indicate that ethanol has differential effects on fetal weight and skeletal development, and that the skeletal sites differ in their sensitivity to ethanol. [2] Overall, it can be concluded that adverse effects from the effects of ethanol treatment are only seen at very high doses only relevant to deliberate and repeated oral consumption of ethanol. The most important studies are the 2-generation study which shows a NOAEL of 13.8g/kg and the inhalation studies that show a NOAEC of 16000ppm (the maximum tested exposure, which is close to or exceeding 50% of the lower explosive limit.) On this basis, it can be concluded that it is impossible to reach the doses of ethanol required to produce any sort of adverse reproductive response other than by repeated oral consumption of large amounts of ethanol, doses normally only associated with problem drinking, and therefore classification for reproductive or developmental toxicity in the context of a chemical substance is not appropriate or warranted. [2] |
|
Skin, eye and respiratory irritations |
Skin: No adverse effect observed (not irritating) [1] Eye: Adverse effect observed (irritating) [1] |
|
Metabolism: absorption, distribution & excretion |
No bioaccumulation potential [1] Absorption values: Oral: 90 %, Dermal: 21 %, Inhalation: 75 % [1] |
|
Exposure limits |
DNEL: 950 mg/m³ (workers, inhalation, long term, systemic effects, carcinogenicity) DNEL: 114 mg/m³ (general population, inhalation, long term, systemic effects, carcinogenicity) DNEL: 1 900 mg/m³ (workers, inhalation, acute/short term, local effects, irritation (respiratory tract) DNEL: 950 mg/m³ (general population, inhalation, acute/short term, local effects, irritation (respiratory tract) DNEL: 343 mg/kg bw/day (workers, dermal, long term, systemic effects, repeated dose toxicity) DNEL: 206 mg/kg bw/day (general population, dermal, long term, systemic effects, repeated dose toxicity) DNEL: 87 mg/kg bw/day (general population, oral, long term, systemic effects, repeated dose toxicity) [1] |
|
Drinking water MAC |
|
|
Other information |
Skin sensitisation: No adverse effect observed (not sensitising) Respiratory sensitisation: No adverse effect observed (not sensitising [1] Neurotoxicity: Adverse effect observed (subchronic, rat, inhalation route) [1] There is no specific classification for the neurotoxicity end point. Developmental neurotoxicity effects are seen only at very high doses, insufficient to trigger classification. Similarly, repeat dose effects are only seen at concentrations well above the levels required for STOT(RE) classification. Acute effects in humans are only seen at high concentrations and are subtle. They are not considered to be sufficient to trigger a STOT(SE) classification. [2] |
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
LD50: 8 300 mg/kg bw (oral route, mouse), No adverse effect observed [1] |
|
Chronic toxicity (NOEL, LOEL) |
NOAEL: 9 400 mg/kg bw/day (subchronic, mouse, Oral route - systemic effects, repeated dose toxicity) [1] NOAEL: 20 700 mg/kg bw/day (subchronic, mouse, oral route) [1] NOAEC: 30 400 mg/m³ (subchronic, rat, inhalation route) [1] NOAEC: 19 000 mg/m³ ((subchronic, rat, inhalation, neurotoxicity) - Adverse effect observed [1] NOAEC: 40 000 mg/m³ (subchronic, rat, inhalation, immunotoxicity) - No adverse effect observed [1] Rats: No effect level >3000mg/kg [2] NOAEL>44000mg/kg (mice, female, cancer) [2] NOAEL>4250mg/kg (mice, male, cancer, based on historic control data) [2] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
| REACH/CLP |
Danger! According to the harmonised classification and labelling (CLP00) approved by the European Union, this substance is a highly flammable liquid and vapour. Additionally, the classification provided by companies to ECHA in REACH registrations identifies that this substance causes damage to organs, is toxic if swallowed, may cause cancer, is toxic in contact with skin, is toxic if inhaled, causes serious eye damage and causes skin irritation. Additionally, the classification provided by companies to ECHA in CLP notifications identifies that this substance causes serious eye irritation. [1]
According to REACH registrations: H225: Highly flammable liquid and vapour. H319: Causes serious eye irritation. H371: May cause damage to organs. H302: Harmful if swallowed. H350: May cause cancer. H315: Causes skin irritation. H370: Causes damage to organs. H301: Toxic if swallowed.
According to CLP notifications: H225: Highly flammable liquid and vapour. H319: Causes serious eye irritation. H371: H302: Harmful if swallowed.
Properties of concern: There is no overall agreement among data submitters, but a minority indicate they consider this substance as Carcinogenic (14.28% of REACH registrations). Of the minority indicating the property of concern, most indicate that it may relate to an impurity or additive rather than the substance itself. [1] |
|
EINECS regulation |
̵listed on EINECS (European INventory of Existing Commercial chemical Substances) List |
|
OSHA regulations etc. |
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
|
|
|
CREATED, LAST UPDATE |
|
|
Created |
2019. 12. 03 |
|
Last update |
2020. 06. 02 |
|
REFERENCES |
|
|
[1] ECHA, Ethanol, https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.000.526, Accessed 2019. 12. 03 [2] ECHA, Ethanol, https://echa.europa.eu/hu/registration-dossier/-/registered-dossier/16105/7/8, Accessed 2020. 06. 02 [3] PUBCHEM, Ethanol, https://pubchem.ncbi.nlm.nih.gov/compound/Ethanol#section=Ecotoxicity-Values, Accessed 2020. 06. 02 [4] American Conference of Governmental Industrial Hygienists TLVs and BEIs. Threshold Limit Values for Chemical Substances and Physical Agents and Biological Exposure Indices. Cincinnati, OH 2017, p. 30 Accessed 2020. 06. 02 |
|