|
|
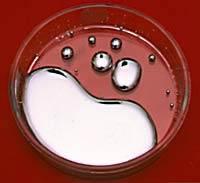
Mercury is the only common metal which is liquid at ordinary temperatures. Mercury is sometimes called quicksilver. It is a heavy, silvery-white liquid metal. It is a rather poor conductor of heat if compared with other metals but it is a fair conductor of electricity. It alloys easily with many metals, such as gold, silver, and tin. These alloys are called amalgams. The most important mercury salts are mercuric chloride HgCl2 (corrosive sublimate - a violent poison), mercuric chloride Hg2Cl2 (calomel, still used in medicine occasionally), mercury fulminate (Hg(ONC)2, a detonator used in explosives) and mercuric sulphide (HgS, vermillion, a high-grade paint pigment). Applications Mercury metal has many uses. Because of its high density it is used in barometers and manometers. It is extensively used in thermometers, thanks to its high rate of thermal expansion that is fairly constant over a wide temperature range. Its Its ease in amalgamating with gold is used in the recovery of gold from its ores. Industry uses mercury metal as a liquid electrode in the manufacture of chlorine and sodium hydroxide by electrolysis of brine. Mercury is still used in some electrical gear, such as switches and rectifiers, which need to be reliable, and for industrial catalysis. Much less mercury is now used in consumer batteries and fluorescent lighting, but it has not been entirely eliminated. Mercury compounds have many uses. Calomel (mercurous chloride, Hg2Cl2) is used as a standard in electrochemical measurements and in medicine as a purgative. Mercuric chloride (corrosive sublimate, HgCl2) is used as an insecticide, in rat poison, and as a disinfectant. Mercuric oxide is used in skin ointments. Mercuric sulphate is used as a catalyst in organic chemistry. Vermilion, a red pigment, is mercuric sulphide; another crystalline form of the sulphide (also used as a pigment) is black. Mercury fulminate, Hg(CNO)2, is used as a detonator. |