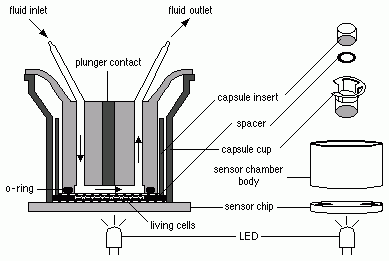
The CM test method is a cytotoxicity and cell-function based in vitro assay that is performed on a sub-confluent monolayer of adherent cells (mouse L929 fibroblasts) cultured on a transwell polycarbonate insert with a porous membrane, which functions as electrode, and a light-addressable potentiometric sensor detecting changes in pH (acidity). Mechanistically, the CM test method is intended to model the cytotoxic action of an irritant chemical on the cell membranes of the corneal and conjunctival epithelium where the test chemical would reside in an in vivo exposure.
The CM estimates the metabolic rate (glucose utilization rate) of a population of cells maintained in low volume flow-through chambers by measuring the rate of excretion of acid by-products and the resulting decrease in pH of the surrounding medium. The metabolic rate is determined indirectly by the number of protons excreted into the low buffer medium (change in pH) per unit time. The light-addressable potentiometer forms the bottom of the flow through chamber and serves as a very sensitive and stable pH meter.
During the course of an experiment, test samples, prepared as dilution series of a test chemical, are introduced in order of increasing concentration to flow-through chambers containing the cells. Therefore, in the CM test method the same cell population is exposed progressively to increasing concentrations of the test chemical. The cells cultured in the chamber are exposed to the test chemical for a short period of time, followed by a rinse step with medium to remove the test chemical. Finally the flow is stopped and the change in pH is measured. All rate of acidification measurements are made on washed cells. These three steps are repeated with increasing concentrations of the test chemical until the highest testable concentration has been used or the metabolic rate has declined to effectively zero.
The rate of change in pH per unit time becomes the metabolic rate of the population of cells. If a test chemical causes cytotoxicity to this population of cells it is assumed that the metabolic rate will fall. A transient up-regulation of glucose metabolism can occur if the cells need energy to maintain their integrity in the face of a mild biochemical insult, but it soon falls below the basal level if exposure to the cytotoxic chemical is prolonged or intensified (higher concentration). The concentration of test chemical that leads to a 50% decline in the basal metabolic rate of the population (MRD50) is the parameter used to measure the cytotoxic effect of the test chemical on the test system (L929 mouse fibroblast cells). The MRD50 value (mg/mL) for each test chemical is calculated from a concentration response curve, and is used to provide a measure of the ocular irritancy potential of the test chemical.