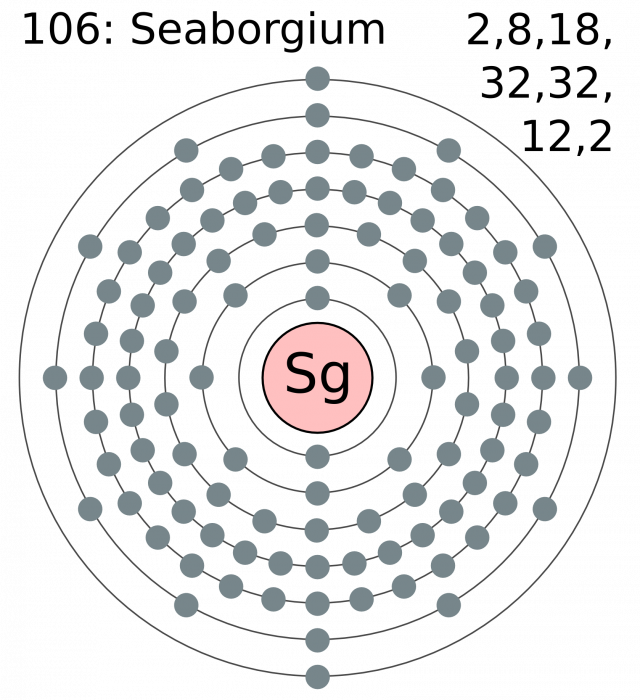
Atomic number 106
Atomic mass 262.94 g.mol -1
Isotopes 1
Electronic shell [ Rn] 7s2 5f14 6d4
Discovered by Albert Ghiorso in 1974
Seaborgium is an artificially produced radioactive chemical element, it's appearance is unknown, it probably has a silvery white or metallic gray colour. The most stable isotope Sg 271 has an half life of 2.4 minutes.
The little research that has been carried out on seaborgium's chemistry suggests that it prefers oxidation state VI and forms an oxy-anion SgO42- and a compound SgO2Cl2, which is entirely in line with its positions in group 6 of the periodic table.
Applications
Saeborgium does not have any known application and little is known about it.