CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
|
|
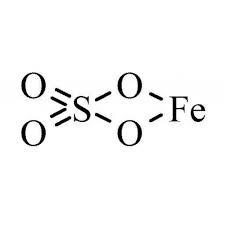
Chemical name |
Iron sulfate |
|
Synonyms |
Ferrous sulfate, Green vitriol, Iron vitriol, Copperas, Melanterite, Szomolnokite |
|
IUPAC name |
Iron(II)sulfate or iron(2+) sulfate |
|
CAS No |
7720-78-7 iron(II)sulfate anhydrous 17375-41-6 iron(II)sulfate monohydrate 7782-63-0 iron(II)sulfate heptahydrate |
|
REACH registration number |
− |
|
EC No |
231-753-5 iron(II)sulfate anhydrous |
|
Molecular formula |
iron(II) sulfate anhydrous FeO4S iron(II) sulfate monohydrate FeH2O5S iron(II) sulfate heptahydrate FeH14O11S |
|
Substance group/chemical family |
Heavy metal salts |
|
Appearance Physical state Odour Form Colour |
solid odourless crystalline pale green |
|
USES AND HANDLING ISSUES |
|
|
Relevant identified uses |
Used in fertilisers, pH regulators and water treatment products, laboratory chemicals, water treatment chemicals, plant protection products, fillers, putties, plasters, modelling clay and metal surface treatment products. This substance has an industrial use resulting in manufacture of another substance (use of intermediates). This substance is used in the following areas: agriculture, forestry and fishing, building & construction work, scientific research and development and formulation of mixtures and/or re-packaging. [2] Also used in iron electroplating baths; in fertilizer; as food and feed supplement; in radiation dosimeters; as reducing agent in chemical processes; as wood preservative; as weed killer; in prevention of chlorosis in plants; in other pesticides; in writing ink; in process engraving and lithography; as dye for leather; in etching aluminum; in water treatment; in qualitative analysis ("brown ring" test for nitrates); as polymerization catalyst. Veterinary uses: In iron deficiency, astringent. [8] Used in metallurgy, and leather tanning. [9] |
|
Handling considerations |
Wear a mask if dust is present. [5] |
|
PHYSICO-CHEMICAL PROPERTIES |
|
|
Molecular weight |
iron(II) sulfate anhydrous 151.8 g/mol iron(II) sulfate monohydrate 169.9 g/mol iron(II) sulfate heptahydrate 277.8 g/mol |
|
Bulk density/Specific gravity |
3.56 g/cm3 [6] |
|
pH |
not applicable |
|
EC |
not applicable |
|
Melting point |
90 °C (iron(II)sulfate heptahydrate) |
|
Boiling point |
300 °C (iron(II)sulfate heptahydrate) |
|
Flash point |
not applicable |
|
Flammability |
not applicable |
|
Vapour density |
not applicable |
|
Vapour pressure |
< 10-5 Pa at 20°C (not applicable) |
|
Solubility in water |
256−266 g/L at 20 °C [1] 486 g/L at 50 °C [1] |
|
Solubility in organic solvents |
not soluble in non-polar organic solvents [1] |
|
Hydrolysis |
It is soluble in water and gets hydrolyzed to give acidic salt solution as it is salt of strong acid and weak base. [1] |
|
Ionicity in water |
Iron(II)sulfate is a salt that completely dissociates in water. [1] |
|
Surface tension |
Not relevant. Iron(II)sulfate is a fully dissociating salt and thus it is not expected to be surface active. [1] |
|
Dispersion properties |
not relevant [1] |
|
Stability and reactivity |
|
|
Chemical stability |
Stable under ordinary conditions [5] |
|
Reactivity hazards |
Iron(II)sulfate does not contain chemical groups that can be ignited with a flame. [1] |
|
Corrosivity |
Considering the chemical structure of ferrous sulfate, oxidising properties are not expected. [1] |
|
Polimerization |
not relevant |
|
Incompatibility with various substances |
Incompatible with alkalies, sol carbonates, gold and silver salts, lead acetate, lime water, potassium iodide, potassium and sodium tartrate, sodium borate, tannin, vegetable astringent infusions and decoctions. [4] |
|
Special remarks on reactivity |
May ignite on contact with arsenic trioxide and sodium nitrate. Potentially explosive reacition with methyl isocyanoacetate at 25 °C. [3] |
|
Physical, chemical and biological coefficients |
|
|
Koc |
KOC (L/kg): Fe2+, SO42- = 0 [1] |
|
Kow |
In non-polar organic solvents the salt iron(II)sulfate is not soluble. Therefore, log Kow was not determined. [1] |
|
pKa |
Fe2+: 6,74 |
|
Henry-constant |
< 6 x 10-7 Pa m3 mol-1 [1] |
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
||
|
Artificial pollution sources |
Due to the applications listed under the section of relevant identified uses. |
|
|
General terrestrial fate |
Iron sulfate is an inorganic salt that dissociates in the soil solution to iron- and sulfate-ions. Both iron- and sulfate-ions are naturally occurring components of terrestrial ecosystems. At agriculturally relevant pH values, nearly all sulfate present is in the soil solution, while the concentration of dissolved Fe in the soil solution is rather low, due to the low solubility of its oxide/hydroxide forms. [1] |
|
|
General aquatic fate |
Iron sulfate is highly soluble in water and, when dissolved in water, it readily dissociates to iron- and sulfate ions. [1] |
|
|
General atmospheric fate |
Not applicable. |
|
|
General persistence and degradability |
It readily dissociates to iron- and sulfate ions. [1] |
|
|
Abiotic degradation and metabolites |
It readily dissociates to iron- and sulfate ions. [1] |
|
|
Biodegradation and metabolites |
Not applicable to inorganic salts |
|
|
Bioconcentration |
Log POW -0.37 |
|
|
Volatilization |
Not applicable |
|
|
Photolysis |
Not applicable |
|
|
Hydrolysis |
Not applicable |
|
|
Soil adsorption and mobility |
Sulfate: Mobile in soils and readily leached. Sorption is pH dependent: sorption increases with decreasing pH. Above pH 6 all sulfate is found in solution. |
|
|
|
Iron: Under typical aerobic environmental conditions (pH 5 – pH 9), the highly soluble Fe (II) salts will be rapidly oxidised to less soluble Fe (III) oxides and hydroxides. Due to the low solubility of the oxide/hydroxide forms, the concentration of dissolved Fe in the soil solution is rather low (< 0.01 - 0.5 mg/L). |
|
|
ENVIRONMENTAL CONCENTRATIONS |
||
|
Measured data |
||
|
ECOTOXICOLOGICAL INFORMATION |
||
|
General adverse effects on ecosystem |
||
|
Acute toxicity (LC50, EC50) |
||
|
Aquatic systems
Terrestrial systems |
Brachydanio rerio: 96 hr (semi-static) Mortality, nom LC50=181.1 mg FeSO4/L [1] Oncorhynchus mykiss:96 hr (flow-through) Mortality, nom LC50=45.1 mg FeSO4/L [1] Daphnia magna: 48-hour (semi-static) Mortality, EC50=31.2 mg FeSO4/L [1] Anabaena variabilis: 96 h (static) Biomass: EC50=36.2 mg FeSO4/L; Growth rate: ErC50 >76.6 mg FeSO4/L Chlorella vulgaris: 72 h (static) Growth rate: ErC50=22 FeSO4 mg /L [1] Selenastrum capricornutum: 72-hour static Biomass and growth rate EC50 > 23.8 mg FeSO4/L [1] Lemna gibba: 7 d (semi-static) Biomass, growth rate and frond number EC50 >103.4 mg FeSO4/L [1] Earthworms (Iron sulphate heptahydrate): LC50(14 days) > 3829 mg FeSO4/kg soil. [1] |
|
|
Chronic toxicity (NOEC, LOEC) |
|
|
|
Aquatic systems
Terrestrial systems |
Oncorhynchus mykiss: 21 d (semi-static) Growth nom NOEC=3.4 mg FeSO4/L [1] Daphnia magna: 21 d (semi-static) Reproduction, NOEC < 1.08 mg FeSO4/L [1] Earthworms (Iron sulphate heptahydrate): NOEC < 70 kg FeSO4/ha. [1] |
|
|
HUMAN HEALTH EFFECTS and PROTECTION |
||
|
Routes of human exposures |
From the molecular structure (inorganic salt), it is suggested that it is unlikely that significant amounts of ferrous sulfate (Fe(II)SO4) can be resorbed through intact skin. The vapour pressure of the inorganic salt is negligible at ambient temperature. Therefore, no toxicologically relevant exposure occurs. [1] |
|
|
General effects |
Acute intoxications [1]: - vomiting - GIT bleeding - diarrhoea - liver dysfunction Chronic intoxication: - liver / haemosiderosis / cirrhosis - spleen / haemosiderosis - kidney / renal failure GIT / fibrosis Mice: liver and spleen / haemosiderosis |
|
|
Endocrine disruption |
||
|
Mutagenicity Genotoxicity |
No data Overall, no genotoxic potential based on a range of in vitro and in vivo studies including negative in S. typhimurium point mutation assay; genetic analysis for induction of diploid and aneuploid cells in Saccharomyces cerevisiae; and negative in vivo micronucleus assays in 57 BL mice and in male ddY mice at dose levels of up to 180 mg/kg bw. Positive finding in in vitro chromosomal aberration study in Chinese hamster fibroblasts. [1] |
|
|
Carcinogenicity |
No carcinogenic potential |
|
|
Reprotoxicity Developmental toxicity |
No data Reduced body weight in dams at 1200 mg/kg bw/day. Increase in dams with no live fetuses and increased implantation loss at 1200 mg/kg bw/day. Relevant maternal NOAEL: 380 mg/kg bw/day FeSO4 – rat; 380 mg/kg bw/day FeSO4 – mice Relevant developmental NOAEL: 380 mg/kg bw/day FeSO4 – rat; 380 mg/kg bw/day FeSO4 – mice [1] |
|
|
Teratogenicity |
No data |
|
|
Skin, eye and respiratory irritations |
Skin irritation: Irritant. Classified with R38 (human data – precautionary, based on physical properties, only one old reference with other citations in literature as moderate irritant without further evidence. Eye irritation: Irritant (R36) (human data – precautionary, based on physical properties,) Skin sensitisation: Non-sensitising [1] |
|
|
Metabolism: absorption, distribution & excretion |
Rate and extent of oral absorption: Rapidly absorbed (10 % up to 60 % in case of iron deficiency) within 2 to 6 hours. Rate and extent of excretion: 0.01 to 0.02 % of the absorbed iron are excreted daily. Distribution: Uniformly distributed via blood. Greatest concentrations in liver, bone marrow, and spleen. Potential for accumulation: Excessive iron is stored in liver, endocrine organs (pancreas) and spleen. Fe2+ and Fe3+ can be converted into each other. Most iron is bound to proteins such as haemoglobin, myoglobin, ferritin, and haemosiderin. [1] |
|
|
Exposure limits |
Rat LD50 oral: 1185 – 1750 mg/kg bw FeSO4 (R22) ‘Harmful if swallowed’ [1] |
|
|
Drinking water MAC |
EPA 300 µg/L /Iron/ [10] |
|
|
Other information |
No potential to induce neurotoxicity [1] |
|
|
Animal toxicity data |
||
|
Acute toxicity (LD50) |
||
|
Chronic toxicity (NOEL, LOEL) |
Oral NOAEL: 90-day study in mice: 120 ppm (100 mg/kg bw/day FeSO4) |
|
|
|
ADI: 0.8 mg/kg bw/day (ferrous iron) derived from human intakes AOEL: 0.4 mg/kg bw/day (Ferrous iron) - Derived from human intakes, supported by teratogenicity study in mice and rats AOEL: 1.3 mg/kg bw/day (sulfate ion) – Human data [1] |
|
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
||
|
EINECS regulation |
̵ |
|
|
OSHA regulations etc. |
Hazardous by definition of Hazard Communication Standard (29 CFR 1910.1200) |
|
|
OTHER INFORMATION, SPECIAL REMARKS |
||
|
Classification and proposed labelling with regard to toxicological data |
R22 – ‘Harmful if swallowed’ (based on data) R38 – ‘Irritating to skin’ (precautionary – indicated in human data but with limited characterisation of effect) R36 – ‘Irritating to eyes’ (precautionary – indicated in human data but with limited characterisation of effect). [1] |
|
|
CREATED, LAST UPDATE |
||
|
Created |
6th of April, 2018 |
|
|
Last update |
6th of April, 2018 |
|
|
REFERENCES |
||
|
[1] European Food Safety Authority (EFSA). Conclusion on the peer review of the pesticide risk assessment of the active substance iron sulfate. Italy, Parma. 2012. [2] European Chemicals Agency (ECHA). Substance information. Accessed from: https://echa.europa.eu/substance-information/-/substanceinfo/100.028.867. Cited: 06.04.2018. [3] [Lewis, R.J. Sax's Dangerous Properties of Industrial Materials. 9th ed. Volumes 1-3. New York, NY: Van Nostrand Reinhold, 1996., p. 1644] [4] [Budavari, S. (ed.). The Merck Index - Encyclopedia of Chemicals, Drugs and Biologicals. Rahway, NJ: Merck and Co., Inc., 1989., p. 635] [5] TOXNET – Toxicology Data Network. https://toxnet.nlm.nih.gov/cgi-bin/sis/search2/f?./temp/~ddSkbD:3 [6] [Lide, DR (ed.). CRC Handbook of Chemistry and Physics. 81st Edition. CRC Press LLC, Boca Raton: FL 2000, p. 4-65] [7] NIOSH. NIOSH Pocket Guide to Chemical Hazards. DHHS (NIOSH) Publication No. 97-140. Washington, D.C. U.S. Government Printing Office, 1997., p. 366] [8] [O'Neil, M.J. (ed.). The Merck Index - An Encyclopedia of Chemicals, Drugs, and Biologicals. 13th Edition, Whitehouse Station, NJ: Merck and Co., Inc., 2001., p. 717] [9] Kirk-Othmer Encyclopedia of Chemical Technology. 4th ed. Volumes 1: New York, NY. John Wiley and Sons, 1991-Present., p. V14: 873 (1995)] [10] USEPA/Office of Water; Federal-State Toxicology and Risk Analysis Committee (FSTRAC). Summary of State and Federal Drinking Water Standards and Guidelines (11/93) To Present |
||