CHEMICAL SUBSTANCE DATASHEET
|
CHEMICAL SUBSTANCE IDENTIFICATION |
||
|
Chemical name |
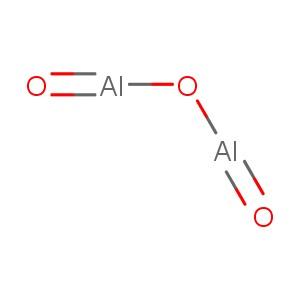
Aluminum Oxide [1] |
|
|
Synonyms |
Aluminium oxide [1], alumina, aloxide, aloxite, or alundum, depending on particular forms or applications [wiki] |
|
|
IUPAC name |
dialuminum;oxygen(2-) [1], oxo[(oxoalumanyl)oxy]alumane [4] |
|
|
CAS No |
1344-28-1 |
|
|
REACH registration number |
|
|
|
EC No |
||
|
Molecular formula |
||
|
Substance group/chemical family |
mono-constituent substance |
|
|
Appearance Physical state Odour Form Colour |
solid odourless crystalline powder white |
|
|
USES AND HANDLING ISSUES |
||
|
Relevant identified uses |
Production of aluminium, manufacture of abrasives, refractories, ceramics, electrical insulators, catalyst and catalyst supports, paper, spark plugs, crucibles and laboratory wares, adsorbent for gases and water vapors, chromatographic analysis, fluxes, light bulbs, artificial gems, heat resistant fibers, food additive (dispersing agent). Activated alumina is/ an effective desiccant for gases and vapors in the petroleum industry. Also used as catalyst or catalyst carrier in chromatography, and in water purification [1] |
|
|
Handling considerations |
HANDLING |
|
|
PHYSICO-CHEMICAL PROPERTIES |
||
|
Molecular weight |
101.961 g/mol [1] |
|
|
Bulk density/Specific gravity |
3.4-4.0 g/cm³ [1] approx. 4 gm/cm3 [4] |
|
|
pH |
|
|
|
Particle size |
0.1 - 150 µm for coarse ground alumina, 0.15 - 250 µm for fine unground alumina, and 0.125 - 40 um for fine ground alumina. [4] |
|
|
EC |
|
|
|
Melting point |
2054 °C [1, 2] 2000 °C [4] |
|
|
Boiling point |
3000 °C [1, 2] 2 980 °C [4] |
|
|
Flash point |
not applicable because the substance is inorganic [4] |
|
|
Flammability |
Not combustible [1] |
|
|
Vapour density |
|
|
|
Vapour pressure |
0 mm Hg (approx) [1, 3], 1 mm at 2158°C [4] |
|
|
Solubility in water |
insoluble [1], 0.00002 g/L at 20 °C [4] |
|
|
Solubility in organic solvents |
Not applicable because the substance is inorganic |
|
|
Solubility in inorganic solvents |
Difficult solubility in mineral acids and strong alkali [1], when heated above 800 °C, it becomes insoluble in acid [1] |
|
|
Hydrolysis |
|
|
|
Ionicity in water |
|
|
|
Surface tension |
because water solubility is below 1 mg/L at 20°C [4] |
|
|
Dispersion properties |
|
|
|
Explosiveness |
there are no chemical groups present in the molecule which are associated with explosive properties |
|
|
Other properties |
Very hygroscopic; electrical insulator, electrical resistivity at 300 °C about 1.2x10+13 ohms-cm; very hard, about 8.8 on Mohs' scale [1] |
|
|
Stability and reactivity |
||
|
Chemical stability |
Stable under normal conditions of use, storage, and transport. [4] |
|
|
Reactivity hazards |
None [4] |
|
|
Corrosivity |
|
|
|
Polimerization |
Ethylene oxide may polymerize violently when in contact with highly catalytic surfaces such as ... the pure oxides of aluminium. [1] |
|
|
Incompatibility with various substances |
None [4] |
|
|
Special remarks on reactivity |
|
|
|
Physical, chemical and biological coefficient |
||
|
Koc |
|
|
|
Kow |
|
|
|
pKa |
|
|
|
log Kp |
|
|
|
Henry-constant |
|
|
|
ENVIRONMENTAL FATE AND BEHAVIOUR |
||
|
Artificial pollution sources |
|
|
|
General terrestrial fate |
|
|
|
General aquatic fate |
|
|
|
General atmospheric fate |
|
|
|
General persistence and degradability |
|
|
|
Abiotic degradation and metabolites |
|
|
|
Biodegradation and metabolites |
According to the guidance given in Annex VII of REACH legislation and Chapter R.7B (Endpoint Specific Guidance) of the ECHA REACH Guidance Document, (2008), the requirements for “Ready biodegradability” can be waived if the substance is inorganic. [4] |
|
|
Bioconcetration |
The available evidence shows the absence of aluminium biomagnification across trophic levels both in aquatic and terrestrial food chains. The existing information suggests not only that aluminium does not biomagnify, but rather that it tends to exhibit biodilution at higher trophic levels in the food chain. [4] |
|
|
Volatilization |
|
|
|
Photolysis |
|
|
|
Hydrolysis |
|
|
|
Soil adsorption and mobility |
|
|
|
ENVIRONMENTAL CONCENTRATIONS |
||
|
Measured data |
|
|
|
ECOTOXICOLOGICAL INFORMATION |
||
|
General adverse effects on ecosystem |
||
|
Acute toxicity (LC50, EC50) |
||
|
Aquatic systems |
The maximum concentration in solution after 7 days was 0.082 mg/L at pH 8 and a loading of 100 mg/L. At pH 6, the maximum concentration in the TDP test solution was 0.005 mg/L. These test concentrations are below the Ecotoxicity Reference Value (ERV) (3.39 mg/L) and therefore aluminium oxide would not classify on an acute basis. Since the loss rate of aluminium from the water column is fast due to the formation of aluminium hydroxide precipitate, there is no concern for long term (chronic) toxicity – this was agreed to by the Classification and Labeling Committee in 1999. Therefore, there is no acute or chronic classification for Al203. [4] | |
|
Terrestrial systems |
|
|
|
Chronic toxicity (NOEC, LOEC) |
||
|
Aquatic systems |
|
|
|
Terrestrial systems |
|
|
|
HUMAN HEALTH EFFECTS and PROTECTION |
||
|
Routes of human exposures |
inhalation, oral |
|
|
General effects |
|
|
|
Endocrine disruption |
|
|
|
Mutagenicity |
|
|
|
Carcinogenicity |
The carcinogenic risk from aluminium and its compounds has not been evaluated by IARC. However, IARC has deemed that that there is sufficient evidence to show that certain exposures occurring during the production of aluminium cause cancer in humans; therefore “aluminium production” has been classified as carcinogenic to humans (Group I) by IARC. [6] |
|
|
Reprotoxicity |
|
|
|
Teratogenicity |
|
|
|
Skin, eye and respiratory irritations |
|
|
|
Metabolism: absorption, distribution & excretion |
|
|
|
Exposure limits |
For workers: inhalation: (DNEL) 15.63 mg/m³ (both long term systemic and local effects) [4] For general population: oral: (DNEL) 3.29 mg/kg bw/day (long term, systemic effects) [4]
|
|
|
Drinking water MAC |
|
|
|
Other information |
|
|
|
Animal toxicity data |
|
|
Acute toxicity (LD50) |
LD50 10 000 - 15 900 mg/kg bw (rat, oral) [4, 5] LC50 (4 h) 888 - 2 300 mg/m³ air (rat, inhalation) [4, 5] LC50 (60 min) 7.6 mg/L air (rat, inhalation) [4, 5] LC0 (4 h) 888 mg/m³ air (rat, inhalation) [4, 5] |
|
Chronic toxicity (NOEL, LOEL) |
NOAEL (rat, oral): 200 - 3 225 mg/kg bw/day [4, 5] NOAEL (rat, oral): 141 - 302 mg/kg diet [4, 5] NOAEL (dog, oral): 1 034 - 1 087 mg/kg bw/day [4, 5] NOAEL (dog, oral): 90 mg/kg diet [4, 5] NOAEC (rat, inhalation): 70 mg/m³ air [4, 5] |
|
ENVIRONMENTAL STANDARDS AND REGULATIONS |
|
| REACH/CLP |
Warning! According to the classification provided by companies to ECHA in REACH registrations this substance is harmful if swallowed and is harmful if inhaled. According to the majority of notifications provided by companies to ECHA in CLP notifications no hazards have been classified.[4] According to REACH registrations: H332: Harmful if inhaled. H302: Harmful if swallowed. According to CLP notifications: H335: May cause respiratory irritation. H370: Causes damage to organs. H372: Causes damage to organs through prolonged or repeated exposure. |
|
EINECS regulation |
̵listed by EINECS (European INventory of Existing Commercial chemical Substances) List [4] |
|
OSHA regulations etc. |
|
|
|
|
|
OTHER INFORMATION, SPECIAL REMARKS |
|
|
Classification and proposed labelling with regard to toxicological data |
|
|
|
|
|
CREATED, LAST UPDATE |
|
|
Created |
2019. 08. 26 |
|
Last update |
2020. 06. 18 |
|
REFERENCES |
|
|
[1] PubChem, Aluminium oxide, https://pubchem.ncbi.nlm.nih.gov/compound/Aluminum-oxide#section=CAS, Accessed 2019.08.26 [2] ILO International Chemical Safety Cards (ICSC), http://www.ilo.org/dyn/icsc/showcard.display?p_version=2&p_card_id=0351, Accessed 2019.08.26 [3] Occupational Safety and Health Administration (OSHA), α-ALUMINA (ALUNDUM), http://www.osha.gov/chemicaldata/chemResult.html?RecNo=242, Accessed 2019.08.26 [4] ECHA, https://echa.europa.eu/hu/registration-dossier/-/registered-dossier/16039/1, https://echa.europa.eu/hu/brief-profile/-/briefprofile/100.014.265, Accessed 2019.08.30 [5] European Commission, ESIS; IUCLID Dataset, Aluminum oxide (1344-28-1) p.61 (2000 CD-ROM edition). Available from, as of April 26, 2010: http://esis.jrc.ec.europa.eu/ Accessed 2019.08.26 [6] IARC (International Agency for Research on Cancer) (1987) Overall Evaluation of Carcinogenicity: An Updating of IARC Monographs (Volumes 1 to 42). Lyon, France: World Health Organization, International Agency for Research on Cancer. |
|